Coal is the fuel that undergoes least human intervention before use: it is burned in essentially the same condition in which it is dug from the ground. Because of this, the term "coal" has a breadth of meaning which reflects the variety of geological conditions under which the fuel is formed. Coal forms only where there is rapidly-growing tropical vegetation, and in a sedimentary basin in which decaying vegetation is periodically covered over with a thick impervious layer of mud, with successive layers becoming deeply buried. This ensures that the organic material is protected from oxidation, and subject to high pressure and, to some degree, elevated temperature. The continued application of pressure causes a sequence of reactions referred to as “coalification”, and the degree to which this process takes place defines the nature of the coal. The degree of alteration is referred to as “rank”, high-rank coals being those most altered. See a separate article for a discussion of this.
The main starting material for the process is wood, consisting of a mixture of cellulose [(C6H10O5)n], lignin [roughly (C10H12O3)n] and a certain amount of inherent moisture. Taking these in a typical ratio 6 : 2 : 1, the dry basis analysis is C 50.00%, H 6.34% and O 43.66%. Coalification takes place by the progressive removal of the elements of carbon dioxide and water into the surrounding rock, lowering the oxygen content, and reducing the amount of volatile matter (VM). The cyclic structures become increasingly polycyclic aromatic. Sulfur and nitrogen from the surroundings infiltrate the structure. The earliest members of the sequence recognisable as coal are the brown coals and lignites. These are soft and woody in texture. Successive stages are sub-bituminous, bituminous, semi-anthracite (“steam coal” in British parlance) and anthracite, with pure carbon in the form of graphite as the theoretical end-member. Broadly speaking, hydrogen content enhances the calorific value of the coal, while oxygen content diminishes it. Because they retain some hydrogen although the oxygen is largely gone, the steam coals have the highest calorific value.
The lower-rank coals are not much represented in Britain. Bituminous coals make the best coke and are in any case the most common, and these are most often used in the cement industry, although steam coals are occasionally used. When burning a pulverised fuel, higher volatiles are preferred because they speed up ignition and allow a coarser grind to be used. Anthracite is too expensive and has insufficient volatiles. Usually, the lowest-priced forms of coal are the finer sizes, and these are most often used, although they tend to have higher free moisture and ash contents.
Britain's coal fields, extensive and with coal seams reaching the surface in many places, have been worked probably from the Neolithic onwards. Coal - probably from the Tyne - was being traded during the Roman occupation. The coal trade rapidly extended due to the depletion of timber during the "Little Ice Age" (1500-1650), and its extention to an international market was the basis - and sole cause - of the Industrial Revolution. It was purely because of this well-developed cheap-energy economy that industrialisation first took place in Britain before it happened anywhere else.
The supply of Tyne coal to London dates from the latter's foundation in the mid first century, and a coastal trade developed with massive fleets of dedicated "collier" ships. Defoe describes a night's storm off Norfolk in 1692 in which more than 200 colliers were sunk. These ships also sailed to the Netherlands, north Germany and Scandinavia. The "chaldron of Tyne coal landed at London" became the standard benchmark of energy price, just as the "barrel of Brent crude" is used today.
Thus there was a well-established coal trade into London at the start of the cement industry. As explained elsewhere, the high-value produce of London had no market on Tyneside, so collier ships ballasted for the return journey with the cheapest available material - chalk from the Thames-side quarries, which were well developed before the cement industry began (see Northfleet history).
Clearly, the development of the cement industry in the London area depended upon this cheap energy supply. London coal gas production began in 1813 and by 1842 was producing 300,000 tons of coke a year, so coke was also readily available, and, being essentially a waste product, was cheaper than coal. Early cement plants used coal for drying slurry and for power generation, and coke for kiln burning. Per tonne of clinker produced, consumption was around 0.5 tonnes of coke and 0.1 tonnes of coal.
Coal could not be used in most static kilns because the fuel was loaded into the kiln with the rawmix, and in the process of gradually raising the temperature, coal would lose its volatiles - and half its calorific value - without ignition. Coal also tended to cause the kiln charge to collapse. With the rise in the relative price of coke towards the end of the nineteenth century, the use of coal was explored. One solution was the Dietzsch kiln, available from the late-1880s. This was a labour-intensive process, and had limited take-up, but the arrival of the rotary kiln in the late 1890s provided a highly productive coal-burning system, and the use of coal escalated rapidly. 50% of heat for drying and burning was provided by coal by 1913, and it was 90% in 1926. It reached 100% in 1943 with the demise of the last static kilns.
Oil displaced coal only for white clinker production until the late 1950s when a proportion of capacity - and most of that of Thames-side - was converted to oil burning, partially in response to a lower oil price, but also as a monopoly-breaking ploy. After this, and brief flirtations with natural gas, coal became emphatically the cheapest kiln fuel from the mid-1970s, and newer kilns had to be retro-fitted with coal grinding equipment. Since then, coal has remained the main fuel, although partially - and at some plants totally - displaced by Petcoke. The increased cost of primary fossil fuels has led to progressive increase, from its beginnings in the late-1970s, of the use of "alternative fuels" such as domestic refuse, landfill gas, tyres, waste lubricants and solvents and so on, although there seems little prospect of these consistently providing the majority of kiln energy.
Coal Tests
The tests used for analysis of coal and similar fuels are specialised and their details are of historical significance, if only because, historically, they have not always been performed correctly in the cement industry.
A lengthy investigation of historic analytical data in the production of the above tables showed that the acceptance of standard testing procedures closely parallels that in the cement industry. Before 1900, people in white coats were very thin on the ground in the coal industry, and the suggestion that a technical issue might be resolved in a laboratory was regarded with extreme suspicion. "Common sense" tests performed by individual coal companies were not comparable on a common basis. The compilers of the Colliery Guardian's Analyses of British Coals and Coke were aware that data presented was not all on a common basis, but since the data were supplied voluntarily, they commented:
it has not been considered advisable to make any alterations in these figures.
The determination of calorific value was almost unheard of. Much of the data quoted was based on fairly flaky equations that made incorrect assumptions about the thermochemistry of coal. In fact, the determination of calorific value, in virtually its modern form, commenced as early as 1877 when the first bomb calorimeter was designed and used by Marcellin Berthelot (1827-1907). A flavour of the attitude of British technologists 40 years later to such scientific techniques can be found in W. E. Dalby's classic text book Steam Power (1st edition 1915). Referring to calorific value determinations (needed to establish the thermal efficiency of steam engines), he says:
When considering the results obtained from fuel calorimeters it must not be forgotten that they are obtained from an exceedingly small quantity of fuel, microscopic, in fact, in relation to the bulk from which the sample was selected. When it is considered what large quantities of coal are purchased by shipping companies and railway companies, even a quantity equivalent to a truck load is not too large to burn as a sample in order to obtain reliable data relating to the calorific value of the fuel. Burnt in the furnace of a boiler, in as nearly as possible the conditions of service, the rate of evaporation of the water in the boiler furnishes data from which an average value of the calorific value of the fuel can be found, and for practical purposes a test of this kind would be preferable to a test in a laboratory calorimeter.
So it's ridiculous to suggest that a 1-gram coal sample could represent the thousands of tons of fuel burned in the inefficient boilers of the time! The "practical" determination of calorific value suggested depends upon a complacent satisfaction with the existing technology and provides no impetus to innovation. It is an example of a recurrent theme in this website - that when energy is ridiculously cheap, who cares about efficiency?
Coal tests fall into two distinct categories:
- Elemental (Ultimate) Analysis
- Proximate Analysis
Elemental Analysis involves basic chemical principles. The fuel is burned, and the resultant gases are analysed in order to derive values for carbon, hydrogen, sulfur, nitrogen and oxygen (the latter being usually obtained by difference). In addition, the mineral analysis of the coal's ash content is routinely performed, typically by xrf analysis of the air-dry coal.
Proximate Analysis is a set of tests designed to characterise the physical nature of the fuel. The tests are all defined in terms of their procedures, and do not necessarily correspond to actual physical reality. The tests are:
Free Moisture: determined by exposing a large sample - typically several kilograms - of lightly-crushed coal, in a thin layer in a wide tray, to ambient air at a temperature below 40°C, until a constant weight is obtained, and determining the loss in weight. The value corresponds roughly with the surface water content that accompanies coal as a result of ash washing and dust suppression. Large samples (100 kg or more) have to be accumulated and protected from water loss during splitting and crushing. The residue of the test is air-dry coal.
This air-dry coal sample is ground, blended and split, usually to leave about 50 g of material below 150 μm, and this is used for the other tests.
Inherent Moisture: determined by heating a few grams of air-dried coal at 105°C for one hour under an atmosphere of nitrogen or argon, and determining the loss in weight. The value corresponds roughly to the content of water contained within the structure - pores and internal surfaces - of the combustible material. In practice, some water is held more tightly than can be released by this treatment, but this is more than counterbalanced by loosely-held combustible material produced during low-temperature decomposition of the coal. If the heating is done under air atmosphere, oxidation considerably increases this decomposition. The sum of the inherent and free moisture values is called the total moisture. It will be noted that it is not possible to obtain a valid total moisture value in a single test. Raw coal can't be ground without losing water from the sample, and inherent moisture can't be expelled in the standard manner without grinding. However, historically, raw coal has often been heated, either leaving some inherent moisture, but usually, by using high temperature, losing a lot of volatile combustible matter.
Ash: determined by heating about a gram of air-dried coal in air, gradually raising the temperature from ambient to a defined maximum (usually 850-950°C) over a period of 1-2 hours, and determining the weight of the residue. It corresponds roughly to the residue that will be obtained when the coal is burned in a furnace. Slow heating of the sample is needed in order to prevent inflaming the sample, which would result in physical loss of sample and loss of alkalis. A high final temperature is needed to complete combustion - although this is never achieved, and the resulting ash always retains a small amount of carbon in encapsulated form. The high temperature results in some loss of the mineral content, notably of alkalis and chloride.
Volatile Matter: determined by heating a gram of air-dried coal, lightly compacted, in a nearly-closed crucible at high temperature (e.g. 925°C) for 7 minutes, then immediately cooling, and determining the loss in weight. The inherent moisture content is deducted to give the volatile matter content. The test is intended to emulate the coking process, and gives the crude gas yield of the coal. The experimental conditions give a repeatable value representing pyrolysis of the coal in the absence of air, but a certain amount of oxidation takes place, raising the value. This is counterbalanced by the fact that pyrolysis is not entirely completed in the test period. The volatile matter is not all combustible, since it includes the mineral volatiles.
Fixed Carbon is simply calculated as the balancing term after moisture, ash and volatiles have been removed. It has no physical reality, because the ash and volatiles are arrived at under different temperature conditions. It corresponds roughly to the coal's ash-free coke yield. Because of the incompleteness of the volatile matter pyrolysis, it is not really all carbon.
Mineral Matter content can in principle be determined by a tedious and exacting process of low temperature solvent extraction, but in practice it is always obtained by some standard calculation. The ash is formed from the mineral matter at high temperature, and various volatile components have been lost. Simple assumptions might be that the CaO and MgO in the ash analysis derive from carbonates, and that the Al2O3 derives from kaolin (2SiO2.Al2O3.2H2O), in which case mineral matter would be given by MM = Ash + 0.785CaO + 1.092MgO + 0.353Al2O3. The intention is to obtain a value approximating the coal's content of inorganic material.
Calorific Value is the fuel's most important - and most abused - property. It is determined by combustion of about a gram of air-dried fuel in oxygen under pressure in an adiabatic bomb calorimeter. The enthalpy of combustion of the fuel is calculated from the temperature rise in the water in which the bomb is immersed, with various corrections for various non-standard reactions that take place - e.g. the burning of sulfur to sulfuric acid rather than sulfur dioxide and the reaction of nitrogen with oxygen to give nitric acid. The result obtained is the "gross" value, in which the combustion products are in their standard state at 25°C: carbon burns to carbon dioxide gas, hydrogen burns to liquid water, sulfur burns to sulfur dioxide gas, and nitrogen remains as N2 gas. In most applications coal combustion actually produces water in vapour form, and the "nett" calorific value is obtained by calculation, deducting from the gross value the latent heat at 25°C of the water formed. The water evaporated is the inherent moisture, plus that formed by burning hydrogen.
CVnett = CVgross - 0.024426(%IM + 8.9364×%H) (MJ/kg).
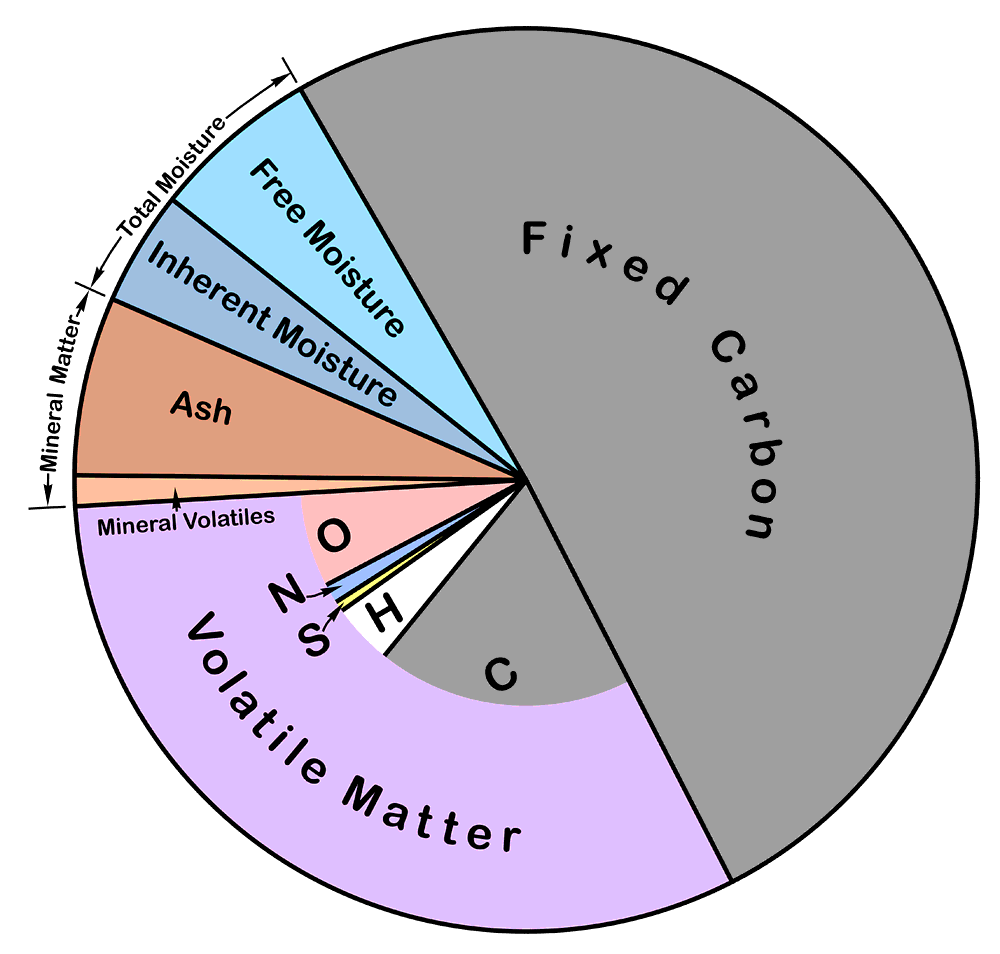
Pie chart of proximate analysis: the inner part of the pie shows the elemental analysis of the combustible part.
In the cement industry, these quantities are all of importance. The moisture content represents water that will have to be evaporated, subtracting from the available energy. Excessive free moisture also causes significant handling problems, meaning that the fuel can't be fed at a uniform rate. The ash content ends up in the product, so its amount and chemical composition can have a profound effect upon the chemistry and uniformity of the clinker. The volatile matter defines the rank of the coal, and affects the rate of combustion and therefore the shape and heat-generating profile of the flame. The rank of the coal is also economically significant because impact strength, compressive strength and resistance to grinding are all at their minimum in the range 20-25% DMF volatile matter, so that grinding costs rise on either side of that range.
Because it represents the energy content of the fuel, the calorific value is the fundamental property from which assessments of the energy-efficiency of the manufacturing process are derived. Before 1918, the calorific value of the fuel was rarely discussed, let alone determined. When serious research on rotary kilns began after that date, fuel started to be analysed in detail, and responsibility for continually gathering data devolved onto individual cement plants. This led to a number of simplifications of test procedure, in which quality of data was sacrificed in favour of quantity. The standard calorific value determination was lengthy and exacting and required (in the early days) considerable mathematics to correct for non-adiabatic conditions.
The process was simplified by using the Roland Wild calorimeter. This oxidised the fuel by mixing with sodium peroxide and firing it in a calorimeter at atmospheric pressure, so the combustion products were sodium carbonate, sodium hydroxide and sodium sulfate. While in theory the result could be calculated back to standard conditions from first principles, in practice the calorimeter was calibrated using a coal sample analysed by the reference method. Because the fuel/peroxide mixture was dangerously explosive if any moisture remained in the fuel, it became the practice to oven-dry the fuel, losing volatiles, so that low results were always obtained, and the extent of the errors was very much a function of the procedures used at each location.
Fuel analysis data is reported in several different ways. The standard reporting form is "as received", in which data obtained on air-dried samples are corrected for the free moisture content. In practice, the basis of reporting is often not specified, and a calorific value may be "as received", "air-dried", "oven-dried" or even "dry, ash-free". For example, a coal might have analyses on different bases as follows:
| As Rec'd | Air Dry | Oven Dry | DAF |
|---|
| Free Moisture | 6.0 | - | - | - |
| Inherent Moisture | 4.1 | 4.36 | - | - |
| Ash | 6.4 | 6.81 | 7.12 | - |
| GCV | 28.63 | 30.46 | 31.85 | 34.29 |
| NCV | 27.58 | 29.34 | 30.68 | 33.03 |
In historic texts (or in modern ones, come to think of it), the exactitude with which the basis of coal analysis is specified is an excellent measure of the competence of the writer.
