Although this page is often accessed as a stand-alone piece, it is part of a work on the history of the British and Irish cement industries, and where statements are made about the historical development of techniques, these usually refer only to developments in Britain.
Portland cement clinker is the essential ingredient of Portland cement. Portland cement is obtained by grinding clinker with only minor amounts of a few other minerals, so its composition does not depart far from that of clinker. Other cements (i.e. non-Portland cements, for example pozzolanic cements, blast furnace slag cements, limestone cements and masonry cements) contain larger amounts of other minerals and have a much wider composition range. Although the other potential ingredients may be cheap natural materials, clinker is made in an energy-intensive chemical process - in a kiln - and its production is the main concern of this website. Between one and two billion tonnes a year of clinker are made world-wide, and the details of its formation are therefore of great economic significance, since no viable alternative ingredients for making cement-like materials currently exist.
Unlike many other thermal products (e.g. aluminium, pig-iron), clinker is a fairly complex mixture of different minerals, and so its production depends on a multi-dimensional control of raw materials and a multi-staged heat treatment. It has been likened to a "man-made igneous rock", and an understanding of its structure and chemistry requires the application of many principles of geochemistry.
Portland Cement Clinker consists essentially of four minerals:
There are also many other non-essential minerals that occur in small quantities.
 Picture: ©Dylan Moore 2011, and licensed for reuse under this Creative Commons Licence. Clinker leaving the cooler on an inclined conveyor to the clinker store. It was pretty hot.
Picture: ©Dylan Moore 2011, and licensed for reuse under this Creative Commons Licence. Clinker leaving the cooler on an inclined conveyor to the clinker store. It was pretty hot.
Clinker produced by early static kilns was in the form of large pumice-like lumps. Rotary kiln clinker on the other hand, because of the rolling action of the kiln, emerges as fairly regular roughly spherical hard nodules of diameter typically 5-50 mm, together with a certain amount of dust abraded from the nodule surfaces.
Clinker minerals react with water to produce the hydrates that are responsible for cement’s setting and strength-giving properties. Reaction with water occurs only at the surface of the clinker particle, and so only proceeds rapidly if the clinker is finely ground to produce a large reaction surface. Un-ground clinker, when exposed to humid air, is hydrated only very gradually, and clinker can be kept in a dry place for several months without appreciable deterioration. It can also be transported from one plant to another in ordinary bulk ships and vehicles, and is traded internationally.
 Clinker Store. Picture: ©NERC
Clinker Store. Picture: ©NERC  Modern clinker store. Two large "first in, first out" silos, holding about a week's production, are needed to cope with today's variable clinker. The size of this one can be gauged from the spiral stairway to the right.
Modern clinker store. Two large "first in, first out" silos, holding about a week's production, are needed to cope with today's variable clinker. The size of this one can be gauged from the spiral stairway to the right.
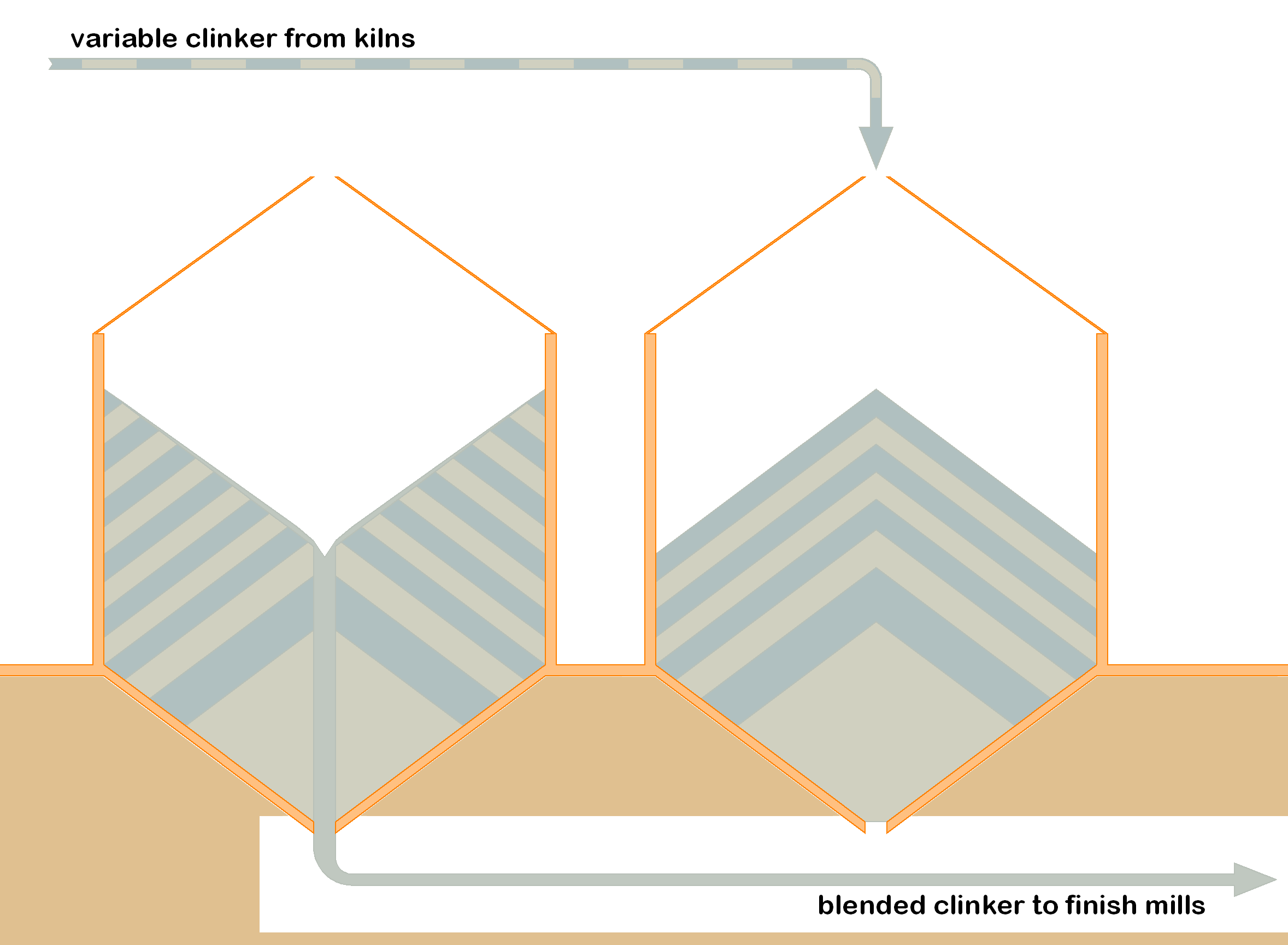
Blending in clinker silos.
Because of its nearly spherical particles, clinker has excellent flow characteristics. Clinker delivered from above naturally produces neat conical piles with a slope angle of about 37°. Because of this, gravity can be used to produce a high degree of blending. On extracting clinker from below a conical pile, "rat-holing" takes place, producing a uniform blend of the variable layers of clinker laid down during the building of the pile. In practice, this is effective for blending out both short-term and medium-term variations. Of course, a minimum of two piles or silos is required for this. If a single silo is used, fresh clinker simply "rat-holes" straight through the silo, and no blending occurs. For a modern 1.5 million tonne per year plant, two silos of about 30 m diameter are required.
Detailed discussions of various aspects of clinker are to be found in the menu items at the top of this page.